Human intracytoplasmic sperm injection (ICSI) technology has been developed for more than 30 years. It was initially mainly used to treat male infertility patients caused by severe oligozoospermia, asthenozoospermia, and teratozoospermia. However, with the development of assisted reproductive technologies such as immature egg in vitro culture, egg freezing, and preimplantation genetic testing, ICSI The usage ratio has increased significantly. Although ICSI technology is relatively mature at present, there are still many details worthy of attention and improvement. In order to standardize and optimize the ICSI operation of practitioners of human assisted reproductive technology, this consensus was compiled by the Reproductive Medicine Professional Committee of the Chinese Medical Doctor Association and jointly with many reproductive medicine centers across the country.
01 Indications of ICSI
01. Male factor
① Extremely severe oligozoospermia and asthenozoospermia, that is, sperm concentration <1×106/mL and sperm forward motility percentage <1%, and the total number of forward motility sperm after treatment is less than 1 million.
② Special types of teratozoospermia, such as globozoospermia, macrocephaly, acephaly and multiple morphological abnormalities of sperm flagella.
③ Abnormal sperm function.
④ Ejaculatory dysfunction.
⑤ Sperm immune factors.
02. Non-male factors
① A PGT cycle is required;
② IVM cycle;
③ Egg freezing and thawing cycle;
④ Complete fertilization failure in the previous conventional IVF cycle for unknown reasons;
⑤ Abnormalities in the zona pellucida are common in eggs from the previous cycle.
ICSI should be considered as the only or preferred method of insemination for both male and non-male factors as mentioned above. For cases with a history of contamination during routine IVF insemination and failure to be cured by antibiotics, there is still a risk of contamination through IVF insemination again, and ICSI can be tried.
02 Normal and common abnormal egg morphology in ICSI cycles
Typically, only nuclear mature eggs with the first polar body (Pb1) present are subjected to ICSI insemination. This consensus summarizes normal and common abnormal egg shapes for reference. See Figure 1 for details.
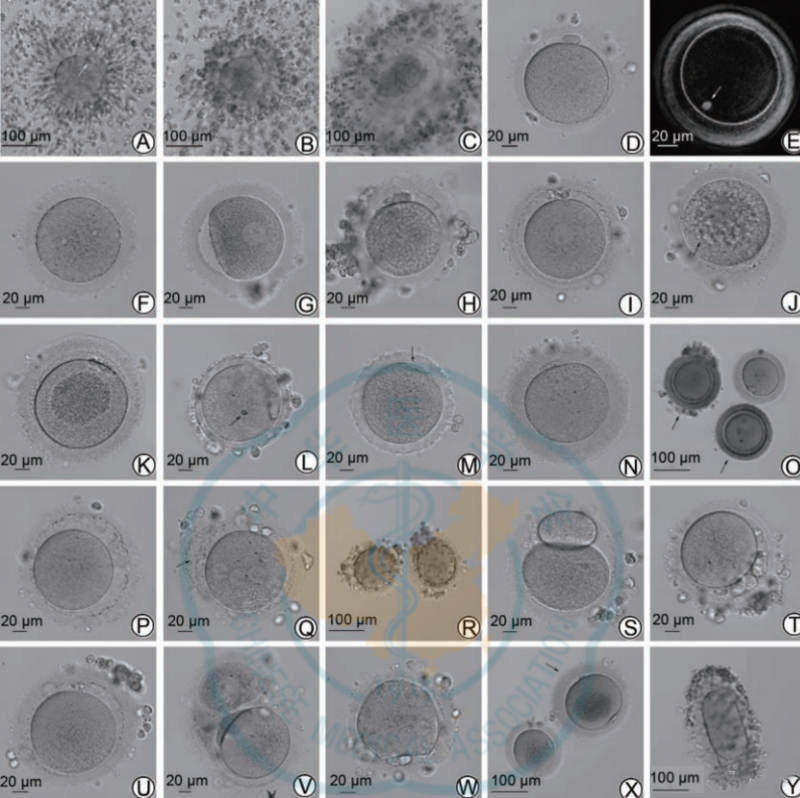
Note: COC indicates the cumulus-oocyte complex; Pb1 indicates the first polar body; ZP indicates the zona pellucida; PVS indicates the perivitelline space
Figure 1 Normal and common abnormal human eggs
A shows COC (mature eggs, cumulus cells are loose, and the arrow indicates Pb1); B shows COC (immature eggs, cumulus cells are dense, and Pb1 is not seen); C shows COC (degenerated eggs and zona pellucida is ruptured); D Shows a normal morphological egg; E shows a normal morphological egg under a polarized light microscope, with the spindle (indicated by the arrow) located just below the polar body; F shows an MⅠ egg; G shows a GV egg; H shows a rough cytoplasm with obvious and uniform granularity; I shows that smooth endoplasmic reticulum clusters can be seen in the cytoplasm, which is as smooth as a disc; J shows that there are multiple vacuoles in the cytoplasm (with a sunken feeling, the largest vacuole diameter is about 16 μm, indicated by arrows); K shows that the cytoplasmic organelles are aggregated in the center The area has obvious granularity; L shows abnormal ZP, which is agar/wax-like, without PVS, and refractive bodies appear in the cytoplasm (arrow, about 5 μm); M shows abnormal ZP, with jagged edges, and Pb1 is crescent-shaped (arrow) shown), no PVS; N indicates ZP thickening (>20 μm is thickening); O indicates ZP coloration and decreased transparency (the arrow indicates the egg); P indicates enlarged PVS (can accommodate at least 2 normal-sized Pb1 ); Q indicates the ZP interlayer (indicated by the arrow); R indicates a large number of particle fragments in the PVS; S indicates giant Pb1 (long diameter about 80 μm); T indicates multiple and larger Pb1; U indicates fragmented Pb1 and increased PVS; V Shows conjoined eggs; W shows irregular membrane; Eggs; Smooth endoplasmic reticulum clusters (SERc), large vacuoles (diameter >14 μm) or multiple small vacuoles, accumulation of cytoplasmic organelles, serrated or waxy zona pellucida, and multiple abnormal egg morphology will affect fertilization, embryonic development and pregnancy. The outcome will have a greater negative impact, but some eggs can still be used to obtain healthy live babies through ICSI. Therefore, it is recommended that these eggs be specially marked and not be used as the first choice for transplantation.
03 Microinjection of eggs
01. Microscopic operating system
The microscopic operating system should be placed in a Class 100 purification area (ISO Class 5). This area should be relatively independent, quiet, and have a small flow of people. It should be far away from the door and transfer window. Try to avoid the air outlet of the high-efficiency filter from blowing directly on the operating table and operators. This way It can ensure a stable operating environment and allow operators to be more focused without being disturbed by external factors. In addition, the incubator related to micromanipulation should be as close as possible to the micromanipulation equipment to increase the smoothness of the operation process and reduce the time that gametes or embryos are exposed to the outside world.
02. Preparation of ICSI operation dish
There is no unified standard for the specific layout of various droplets in the ICSI operating dish. They can be prepared according to personal operating habits, but the following basic principles and reference tips should be followed: ① The operating fluid droplets should be concentrated in the middle of the dish as much as possible to avoid when the operating needle drops. Touch the side wall of the dish; ② If it is extremely oligospermic, weak sperm or testicular/epididymal sperm, you can directly make the sperm suspension into droplets without making PVP droplets; ③ It is recommended that two people make the dish and cover it with culture oil in time , to avoid changing the droplet osmotic pressure due to volatilization.
03. Installation of micromanipulation needle
① Avoid creating a large number of bubbles in the injection needle during installation;
② If the operating needle encounters great resistance when inserting into the needle holder, do not force the insertion. Check whether there is any broken glass residue in the needle holder pipeline;
③ If you find that the operating needle is not blowing smoothly, especially if you cannot stably control the sperm in the injection needle, you should first check the needle installation position and the tightness of the crystal nut; if the above problem is not solved after ensuring that the needle is in place and the crystal nut is tightened, It should be considered that the sealing gasket in the crystal nut is aged or damaged and needs to be replaced in time;
④ Adjust the focus of the installed operating needle under a low-magnification objective lens (4× or 10×), and make the injection needle and the egg-holding needle align in a straight line;
⑤ The angle of the front end of the injection needle is not completely parallel to the bottom of the dish, but the angle should not be too large. The smallest angle that can successfully stop the moving sperm is better.
04. Degranulation of eggs
It is recommended that the egg is exposed to hyaluronidase for <30 s. The total degranulation time is controlled within 1 to 2 minutes, so it is not recommended to operate too many eggs at one time (5 to 8 eggs is appropriate). It is recommended to use sintered glass straws with decreasing caliber (inner diameter) or commercial egg stripping needles to gradually degranulate the eggs: usually first use a Pasteur straw with a smooth mouth to blow and digest in the enzyme, and then use Transfer a pipette with an inner diameter of about 200 μm into clean gamete buffer, then use a pipette/egg needle with an inner diameter of 160~180 μm for "rough disassembly", and finally use a pipette/egg needle with an inner diameter of 135~140 μm to peel off the granulosa cells. Clean; the degranulated eggs should be fully washed and finally moved into cleavage culture medium and incubated for at least 1 hour.
05. Basic steps of microinjection
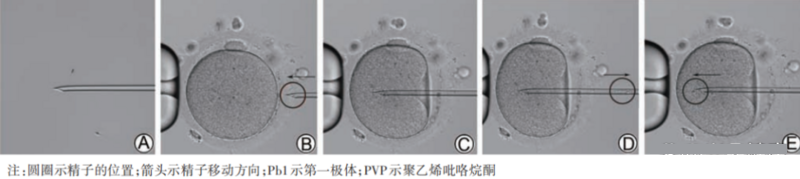
Figure 2 Microinjection process (200×)
A shows that the middle or lower section of the sperm tail is gently pressed to brake; B shows that the position of Pb1 is adjusted and the egg is fixed, and the needle tip is against the zona pellucida and the sperm is moved to the needle tip; C shows that the sperm breaks through the zona pellucida and penetrates into the cytoplasm, but at this time it does not The membrane is not broken; D indicates that when the cytoplasm flows into the needle instantaneously (the membrane is broken), the negative pressure is immediately terminated; E indicates that the sperm enters the cytoplasm, and PVP injection should be avoided.
Injection of sperm into the cytoplasm is key to fertilization. The sperm head and neck slide smoothly out of the needle tip and do not continue to move backward with the rebound of the cell membrane after the needle is withdrawn, which indicates that the sperm has been successfully injected into the cytoplasm. When it is observed that the membrane is broken successfully, but the sperm is obviously blocked during injection and still stays inside the needle tip, and the sperm will slowly move outward along the puncture channel after the needle is withdrawn, it indicates that the "folds" of the regurgitated membrane hinder the entry of sperm. . In this case, the cytoplasm can be sucked back a second time, and then the sperm can be re-injected; or a second puncture can be performed parallel to the first puncture channel, and the membrane can be re-ruptured before the sperm can be injected again. The cell membrane stretched after the first puncture can usually be ruptured with only slight negative pressure or even no negative pressure during the second puncture. It is recommended to perform sperm immobilization and egg injection under 200×~400× magnification.
06. Sperm immobilization
For the braking of sperm, it is enough to successfully prevent the forward movement and tail swing; the sperm should be injected as soon as possible after braking. It is not recommended to brake a sufficient number of sperm in advance before injecting. For patients with extremely severe oligospermia, in order to prevent the eggs from being exposed to the outside world for too long, a sufficient number of sperm can be captured in advance, but no braking is performed in advance.
07. The position of the polar body during ICSI
It is recommended to place Pb1 at 12 o'clock or 6 o'clock, and perform puncture at 3 o'clock or 9 o'clock (for left-handed needle insertion). Use the microinjection needle to gently press the egg zona pellucida to rotate Pb1 to the clearest position on the puncture focal plane.
08. Injection needle penetration depth
It is recommended that the puncture depth be 1/2~2/3 of the diameter of the cytoplasm. However, for eggs that suddenly rupture (no puncture funnel is formed), it is not recommended to continue inserting the needle, and sperm can be directly injected into the cytoplasm.
04 Fertilization observation
Although some studies have reported that healthy babies can be obtained by screening normal parental diploid embryos through PGT, considering the low normal embryo rate and high detection cost, it is recommended to discard ICSI-1PN-derived embryos. Embryos derived from ICSI-0PN may have pronuclei appearing too early or too late and miss the observation time. In view of the fact that embryos derived from high-quality ICSI-0PN have similar normal chromosome incidence, diploid ratio of parents, and pregnancy outcomes as embryos derived from ICSI-2PN, it is recommended that embryos derived from high-quality ICSI-0PN be cultured to form blastocysts that can be used , transplantation or cryopreservation will be performed after full informed consent.
05 Response to special circumstances of ICSI and other details
01. The first meiosis (MI)
Treatment of mid-term eggs
For patients with low egg maturity rate (<50%) or advanced age (≥40 years old), MI eggs can be cultured in vitro for a short period of time (within 8 hours after egg retrieval), thereby increasing egg utilization and the number of available embryos. However, in view of its high aneuploidy rate and adverse pregnancy outcomes, PGT-A testing can be performed on the formed blastocysts if conditions permit.
02. Response to eggs that are prone to degradation
During the degranulation process, high-frequency and large-scale suction should be avoided, and the inner diameter of the egg stripping needle should not be <135 μm; excessive stripping of granulosa cells should be avoided, and the residual granulosa cells should not affect the observation and injection of eggs; when sperm or cytoplasmic adhesion occurs, When the needle is severe, the ICSI needle should be replaced in time; the horizontal angle of the front end of the ICSI needle should not be too large; for eggs with high egg membrane fragility, it is recommended to appropriately extend the incubation time before performing ICSI.
03. Treatment of eggs without zona pellucida
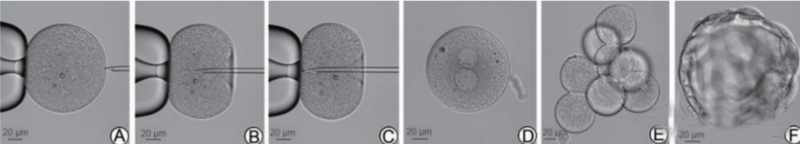
Figure 3 Intracytoplasmic sperm injection and embryonic development of human zona pellucida eggs
A shows the fixed egg; B shows the membrane puncture; C shows the sperm injection; D shows normal fertilization on the 1st day; E shows the 8-cell embryo on the 3rd day; F shows the blastocyst on the 5th day.
During the ICSI process, the holding needle negative pressure should not be too large, and the ICSI needle penetration should not be too shallow; since Pb1 cannot be observed, if possible, a polarized light microscope can be used to confirm the spindle position before ICSI; cleavage stage embryos derived from ZFOs Disintegration is easy to occur during transplantation or freezing, so it is recommended to culture blastocysts; to avoid blastomere decomposition during the medium replacement process, the cleavage stage culture medium in the culture droplet can be directly removed and re-injected into the blastocysts culture medium, or use one-step culture medium; it is recommended to culture ZFOs in single droplets or use a time-lapse imaging system.
04. Response to ICSI complete fertilization failure
AOA should be applied with caution. It is recommended that patients whose previous ICSI fertilization completely failed or whose fertilization rate was low should be judged by cross-over test of patient's sperm and donor egg, mouse egg activation test (MOAT) or PLC ζ staining of sperm.
Spermogenic or ovogenic OAD; AOA can be used to treat cases of type I cyclocephaly or spermogenic OAD; commonly used chemical activators include ionomycin, A23187 and strontium chloride. The comparison of the effects among the three is still controversial, but for patients whose clinical treatment effect is not good when using the above three activators alone, combined application can be tried.
05. Response to completely immobile sperm
During ICSI, it is crucial to distinguish live sperm from dead sperm among completely immobile sperm, because dead sperm cannot activate the egg and normal fertilization occurs. Commonly used methods for identifying viable sperm include artificial sperm activation (ASA), hypotonic swelling test (HOST), laser-assisted immotile sperm selection (LAISS), and sperm tail flexibility test (STFT).
Although the above four methods have their own advantages and disadvantages, IVF laboratories should use at least one of them for ICSI treatment of immotile sperm; given that the safety of ASA is not clear, it should be applied with caution; HOST and LAISS can be used as ASA Alternative methods for invalid cases, but there is no evidence to show which of the two is more advantageous. It can be selected and applied according to laboratory operating habits and equipment conditions; the STFT method can usually be used in combination with the HOST and LAISS methods.
When using HOST, sperm should be exposed to hypotonic fluid for less than 30 s.
06. Sperm selection
There is currently no reliable technology that can completely screen out sperm with DNA fragmentation, and the safety and effectiveness of these technologies are open to question. Therefore, the routine application of the above methods is not recommended; for sperm DNA fragmentation index (DFI) >20%~ 30% of cases with adverse prognostic factors (old age, low ovarian function, repeated implantation failure/miscarriage) can try the above methods for sperm selection, but due to the lack of prospective and random nature of relevant studies, the best method cannot be recommended at present. Sperm selection technology; if DFI continues to be high and repeated ICSI cycles fail more than 2 times, sperm extraction from the testicles can be considered.
07. Other details
(1) Selection of timing for egg incubation, degranulation and ICSI:
Degranulation and ICSI should be performed 2 to 6 hours after egg retrieval or 38 to 42 hours after human chorionic gonadotropin (hCG) triggering; it is recommended to incubate for 1 hour after degranulation before ICSI; patients with poor prognosis can use spindle observation The instrument evaluates egg nucleus-cytoplasm synchrony, and the ICSI time can be appropriately delayed for MIⅡ eggs without spindle.
(2) PVP concentration:
It is recommended to appropriately dilute PVP based on personal technical ability and habits. 5% PVP is a good choice. On the one hand, it reduces the negative impact of high concentrations of PVP on the egg development potential. On the other hand, it hardly increases the duration of the sperm braking operation. .
(3) Hyaluronidase concentration:
Commercial hyaluronidase (80 U/mL) can be applied directly. At the same time, it is recommended that dilution application be carried out without affecting the degranulation effect and time.
Quality control indicators
According to the 2017 Vienna Consensus and the 2018 Expert Consensus on Quality Control of Key Indicators for Chinese Embryo Laboratories, the calculation methods and standards for ICSI laboratory quality control indicators are summarized as follows. See Table 1 for details.
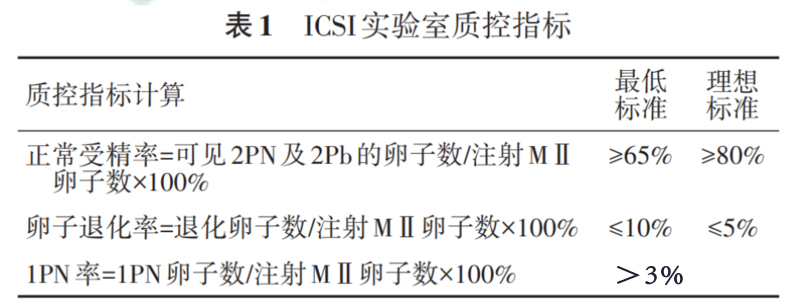
Note: Normal fertilization rate and egg degeneration rate are key quality control indicators; 1PN rate is a general quality control indicator; degenerated eggs are defined as eggs with cytoplasmic disintegration during the ICSI process and apoptosis found during fertilization observation; ICSI shows that within the egg cytoplasm Single sperm injection; PN indicates the pronucleus; Pb indicates the polar body
① Newcomers should fully understand the theoretical basis and influencing factors of ICSI. Only newcomers who are proficient in basic skills such as egg collection, embryo transfer, and egg degranulation can start training for ICSI operations;
② During training, unfertilized or discarded IVM eggs can be used for practice. The patient's eggs can only be processed when the average operation time of each egg (from the start of sperm braking to the end of injection) of the new couple is equivalent to that of the teaching embryologist, the fertilization rate of discarded IVM eggs is >60%, and the egg degeneration rate is <10%. operate;
③ In the initial stage of formal operation of patient eggs, newcomers can inject some of the eggs from patients with a larger number of eggs (>10 eggs). After at least 50 eggs have been injected, when the fertilization rate and egg degradation rate are comparable to the results of the same batch of eggs injected by a senior embryologist, independent operation can be performed.




